Abstract
Introduction: Central venous catheters (CVCs) are ubiquitous in medical practice, however adverse thrombotic complications remain common. Though anticoagulation may be used to prevent or treat catheter-associated thrombosis (CAT), there is an unmet need to safely offset the thrombotic complications of CVCs given the inherent bleeding risks of modern anticoagulants. The contact pathway factors XI and XII, activated by foreign device surfaces, may serve as safe and efficacious therapeutic targets to reduce the burden of CAT while mitigating bleeding complications. In this present study, we evaluated the efficacy of AB023, a novel factor XI antibody inhibitor, in preventing CAT in cancer patients.
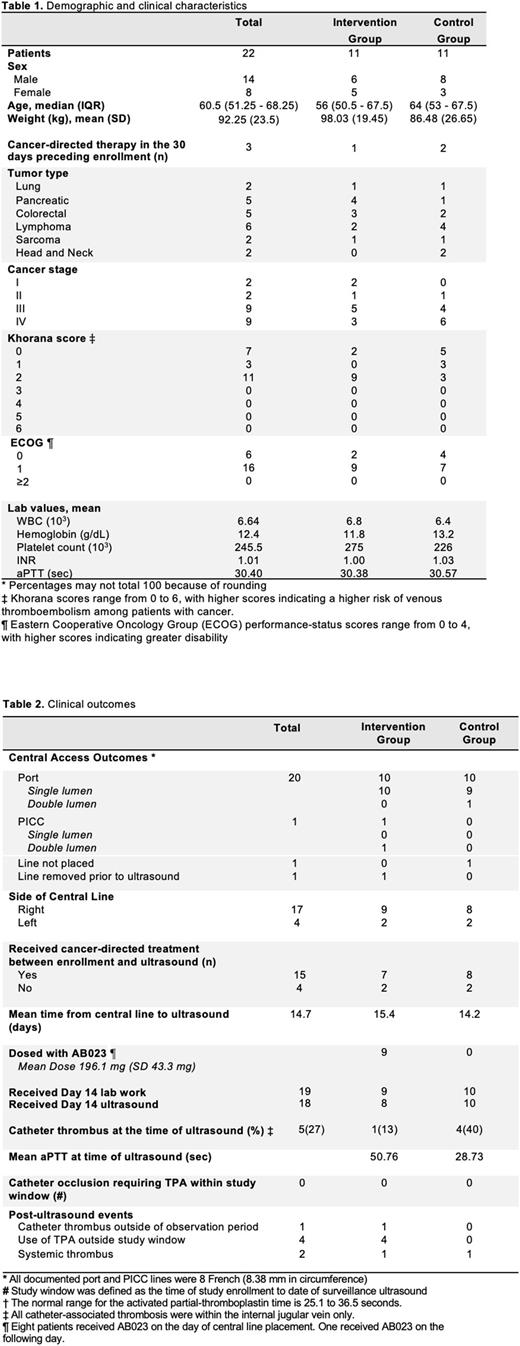
Methods: In this phase 2 trial, we enrolled ambulatory cancer patients over the age of 18 undergoing CVC placement for cancer-directed therapy. Patients enrolled in the interventional study received a single dose of intravenous AB023 (2 mg/kg) administered through the CVC within 24 hours of placement, while those enrolled in a parallel, non-interventional study were merely observed. Patients with acute leukemia, renal or hepatic dysfunction, coagulopathy, moderate or severe thrombocytopenia, or known brain metastases were excluded. Patients underwent surveillance ultrasound on day 14 (± 7 days) following CVC placement to screen for CAT. The primary endpoint was the incidence of total thrombosis at day 14 (± 7) follow up, including CAT detected on surveillance ultrasound within the observation period. Secondary endpoints included change in coagulation parameters (aPTT, PT, INR) compared to baseline, and the incidence of infusion reactions or other drug-related adverse events, and clinically relevant bleeding.
Results: In total, 22 patients (11 in each study) were enrolled. Nine patients in the interventional cohort received AB023, 8 of which underwent surveillance ultrasound, and 10 patients in the control cohort underwent surveillance ultrasound (Table 2). Malignancies included pancreatic cancer (4/11), colorectal cancer (3/11), and lymphoma (2/11) in the interventional study, and lymphoma (4/11) colorectal cancer (3/11), and head and neck cancer (3/11) in the control study. All patients in both the interventional and control studies had a Khorana score of 2 or less. The mean follow-up time was 404 ± 21 days in interventional study and 201 ± 16 days in control study.
The overall incidence of CAT was 1/8 in the interventional study and 4/10 in the control study. AB023 significantly prolonged aPTT in all subjects on day 14 compared to baseline (30.4 sec vs 50.7 sec, p <.001), while no significant change was noted in the control study (30.6 sec vs. 28.8 sec, p .48). INR was not significantly altered in either group compared to baseline. Overall AB023 was well tolerated; there were no infusion reactions, drug-related adverse events, or clinically relevant bleeding. No catheter occlusions requiring alteplase occurred in either group during the first 14 days of observation. Following the observational period, 2 patients developed thrombotic events in the interventional study (1 with pulmonary embolism [Day 99] and 1 with CAT [Day 81]) and 4 patients required alteplase for CVC occlusion. In the control study, 1 patient developed a deep vein thrombosis beyond the study observational period (Day 71). Platelet flow cytometry was performed assessing CD62P and PAC1 expression. There was not a statistically significant difference in platelet activation assessed by mean fluorescence intensity before and following administration of AB023. We will further address the analysis of thrombin-antithrombin complexes, systemic cytokines, D-dimer, and other relevant biomarkers of thromboinflammation.
Discussion In this pilot study of ambulatory cancer patients receiving central line placement, AB023 was well-tolerated without any significant adverse or bleeding related events. Our study found a low incidence of CAT on day 14 ultrasound in AB023-treated patients compared to the published literature and our internal control study. These findings add to a growing body of evidence supporting a critical role for the contact system in device-associated thrombosis, and suggest that targeting the factor XI/factor XII axis could represent a safe intervention to prevent CAT in cancer patients.
Disclosures
Lorentz:Aronora Inc: Current Employment, Current holder of stock options in a privately-held company. Tucker:Aronora, Inc.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties. Shatzel:Aronora, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal